Acids in the Home: Unmasking the Chemistry of Everyday Objects
Related Articles: Acids in the Home: Unmasking the Chemistry of Everyday Objects
Introduction
With great pleasure, we will explore the intriguing topic related to Acids in the Home: Unmasking the Chemistry of Everyday Objects. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Acids in the Home: Unmasking the Chemistry of Everyday Objects

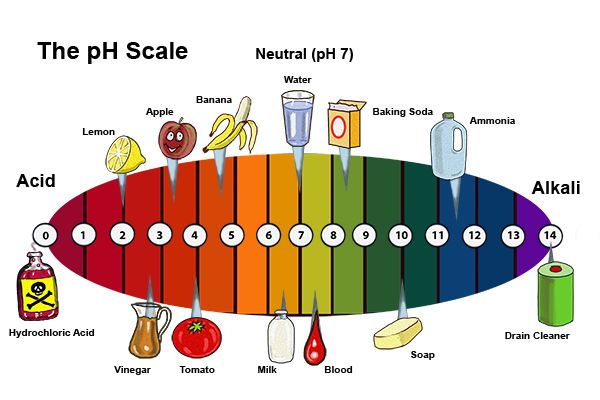
Acids are ubiquitous in our daily lives, often playing a critical role in maintaining cleanliness, enhancing flavors, and facilitating various household tasks. While the word "acid" might conjure images of corrosive liquids in laboratory settings, many common household items contain acids in varying concentrations. Understanding the nature and properties of these acids empowers us to use them safely and effectively while appreciating their diverse applications.
Common Household Acids and Their Applications:
1. Vinegar:
Vinegar, a staple in kitchens worldwide, is a dilute solution of acetic acid (CH3COOH). This weak acid gives vinegar its characteristic sour taste and odor. Its versatility extends beyond culinary uses:
- Cleaning: Vinegar’s acidic nature effectively dissolves mineral deposits, making it an ideal cleaning agent for surfaces like countertops, windows, and even coffee makers. Its ability to neutralize alkaline substances like soap scum and hard water deposits makes it a valuable tool for maintaining a clean and hygienic home.
- Food Preservation: Vinegar’s antimicrobial properties have been utilized for centuries to preserve food. Pickling vegetables and fermenting foods like sauerkraut rely on the acid’s ability to inhibit bacterial growth, extending shelf life and creating unique flavors.
- Pest Control: Vinegar’s acidic nature can deter certain insects, making it a natural and effective alternative to harsh chemical pesticides. Applying vinegar to plants can discourage aphids and other garden pests.
2. Citric Acid:
Citric acid (C6H8O7), found naturally in citrus fruits, is a versatile acid used in various household products. Its tart flavor and ability to act as a chelating agent (binding to metal ions) make it a valuable component:
- Food and Beverages: Citric acid is a common ingredient in soft drinks, candies, and jams, adding a tangy taste and acting as a preservative.
- Cleaning Products: Citric acid’s ability to remove mineral deposits and stains makes it a popular ingredient in cleaning solutions for bathrooms, kitchens, and even dishwashers.
- Cosmetics and Personal Care Products: Citric acid is often used in skin care products as an exfoliating agent and to regulate pH levels. It is also found in some shampoos and hair care products.
3. Ascorbic Acid (Vitamin C):
Ascorbic acid, commonly known as Vitamin C, is an essential nutrient for humans. It is a weak acid found in many fruits and vegetables, particularly citrus fruits.
- Health Benefits: Vitamin C plays a vital role in the body’s immune system, collagen synthesis, and antioxidant defense. It is crucial for maintaining healthy skin, bones, and blood vessels.
- Food Preservation: Ascorbic acid is often added to processed foods as an antioxidant to prevent spoilage and maintain freshness.
- Cosmetics and Personal Care Products: Vitamin C is frequently incorporated into skin care products for its antioxidant and brightening properties.
4. Carbonic Acid:
Carbonic acid (H2CO3) is formed when carbon dioxide dissolves in water. While it is a weak acid, it plays a crucial role in everyday life:
- Carbonated Beverages: The fizz in carbonated drinks is attributed to the presence of carbonic acid, which is formed when carbon dioxide is dissolved under pressure.
- Soil Chemistry: Carbonic acid contributes to the weathering of rocks and the formation of soil. It helps to release essential nutrients from rocks, making them available to plants.
- Biological Systems: Carbonic acid plays a vital role in maintaining the pH balance in the blood and other bodily fluids.
5. Lactic Acid:
Lactic acid (C3H6O3) is produced naturally by bacteria during fermentation. It is a weak acid found in various foods and beverages:
- Dairy Products: Lactic acid is responsible for the sour taste of yogurt, sour cream, and cheese. It is produced by bacteria during fermentation, giving these products their unique flavor and texture.
- Pickles and Sauerkraut: Lactic acid plays a crucial role in the fermentation of pickles and sauerkraut, contributing to their tangy flavor and extending their shelf life.
- Cosmetics and Personal Care Products: Lactic acid is often used in skin care products as a gentle exfoliating agent and to improve skin hydration.
6. Tartaric Acid:
Tartaric acid (C4H6O6) is a naturally occurring acid found in grapes and other fruits. It is widely used in food and beverage production:
- Winemaking: Tartaric acid is responsible for the tartness of grapes and plays a key role in winemaking. It helps to stabilize the wine, prevent microbial growth, and contribute to its flavor profile.
- Baking: Tartaric acid is used as a leavening agent in baking, reacting with baking soda to produce carbon dioxide bubbles, which make cakes and cookies rise.
- Food Additives: Tartaric acid is used as a flavoring agent and a preservative in a variety of food products.
7. Phosphoric Acid:
Phosphoric acid (H3PO4) is a strong acid commonly found in various household items:
- Soft Drinks: Phosphoric acid is a key ingredient in many soft drinks, contributing to their tart flavor and acting as a preservative.
- Fertilizers: Phosphoric acid is a vital component of many fertilizers, providing phosphorus, an essential nutrient for plant growth.
- Rust Removers: Phosphoric acid is used in some rust removers due to its ability to dissolve rust and create a protective coating.
8. Hydrochloric Acid:
Hydrochloric acid (HCl), also known as muriatic acid, is a strong acid found in some household cleaning products:
- Pool Maintenance: Hydrochloric acid is used to adjust the pH of swimming pool water, preventing algae growth and ensuring safe swimming conditions.
- Cleaning Solutions: Hydrochloric acid is found in some toilet bowl cleaners and rust removers.
Understanding the Importance and Benefits of Household Acids:
These acids play a crucial role in various aspects of our daily lives, offering numerous benefits:
- Cleaning and Hygiene: Acids are effective at removing mineral deposits, stains, and grime, contributing to a clean and hygienic living environment.
- Food Preservation and Flavor: Acids are used to preserve food, prevent spoilage, and enhance flavors, making our food supply more diverse and accessible.
- Health and Wellness: Acids like vitamin C are essential nutrients for maintaining good health and supporting various bodily functions.
- Industrial Applications: Acids are essential in various industrial processes, from manufacturing to agriculture, contributing to economic development and technological advancement.
FAQs about Household Acids:
Q: Are all acids dangerous?
A: Not all acids are dangerous. The strength and concentration of an acid determine its potential hazards. Weak acids like vinegar and citric acid are generally safe for household use when handled properly. However, strong acids like hydrochloric acid can be corrosive and require careful handling.
Q: How can I safely use acids in my home?
A: Always follow the instructions on the product label. Wear gloves and eye protection when handling acids, and avoid contact with skin and eyes. Store acids in well-ventilated areas, away from children and pets.
Q: What happens if I mix different acids?
A: Mixing different acids can be dangerous, potentially leading to harmful reactions and releases of toxic fumes. It is crucial to avoid mixing acids unless explicitly instructed in product manuals.
Q: How do I dispose of acidic products safely?
A: Never pour acids down the drain or into the garbage. Check with your local waste management agency for proper disposal methods.
Tips for Using Acids Safely in the Home:
- Read labels carefully: Always follow the instructions on the product label, including safety precautions, dilution recommendations, and storage guidelines.
- Wear protective gear: Use gloves and eye protection when handling acids, especially strong acids.
- Ventilate the area: Work in a well-ventilated area to avoid inhaling fumes.
- Avoid contact with skin and eyes: If acid comes into contact with skin or eyes, immediately flush the area with plenty of water and seek medical attention if necessary.
- Store acids properly: Keep acids in their original containers, tightly sealed, and out of reach of children and pets.
- Dispose of acids responsibly: Follow local regulations for disposing of acidic products.
Conclusion:
Acids are essential components of our everyday lives, contributing to our health, hygiene, and enjoyment of food. Understanding the nature and properties of these acids empowers us to use them safely and effectively. By adhering to safety precautions and using acids responsibly, we can harness their benefits while minimizing potential risks.






Closure
Thus, we hope this article has provided valuable insights into Acids in the Home: Unmasking the Chemistry of Everyday Objects. We hope you find this article informative and beneficial. See you in our next article!