Managing Hydrochloric Acid: Safety Measures, Neutralization, And Spill Response
Managing Hydrochloric Acid: Safety Measures, Neutralization, and Spill Response
Related Articles: Managing Hydrochloric Acid: Safety Measures, Neutralization, and Spill Response
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Managing Hydrochloric Acid: Safety Measures, Neutralization, and Spill Response. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Managing Hydrochloric Acid: Safety Measures, Neutralization, and Spill Response
Hydrochloric acid (HCl), a strong, corrosive acid, is a common chemical used in various industries. It poses significant safety hazards due to its highly reactive nature. Understanding how to handle and neutralize HCl spills is crucial for ensuring safety and mitigating potential risks. This article explores various methods for managing HCl, encompassing safety precautions, neutralization techniques, and spill response protocols.
Understanding Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is a colorless, pungent solution of hydrogen chloride (HCl) in water. Its corrosive properties stem from its ability to donate hydrogen ions (H+) in solution, making it highly acidic. This acidity allows it to react readily with many substances, including metals, carbonates, and organic compounds.
Safety Precautions
Working with hydrochloric acid necessitates strict adherence to safety protocols to prevent accidents and injuries.
-
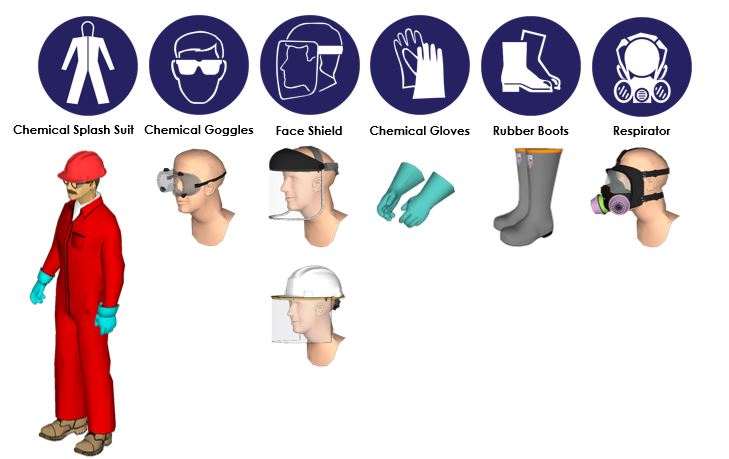
Personal Protective Equipment (PPE): The use of appropriate PPE is paramount. This includes:
- Eye protection: Safety goggles or a face shield are essential to protect the eyes from splashes.
- Respiratory protection: A respirator with acid-resistant cartridges is necessary to prevent inhalation of acid fumes.
- Gloves: Chemical-resistant gloves made of materials like nitrile or neoprene should be worn to protect the skin from contact.
- Protective clothing: A lab coat or apron made of acid-resistant material should be worn to protect the body from accidental spills.
- Ventilation: Adequate ventilation is critical to minimize exposure to acid fumes. Work in well-ventilated areas or use fume hoods.
- Storage: Hydrochloric acid should be stored in a cool, dry, and well-ventilated area, away from incompatible substances.
- Handling: Handle containers carefully to avoid breakage. When transferring HCl, use appropriate pumps or siphons to minimize the risk of spills.
- Emergency Procedures: Ensure that emergency procedures for handling acid spills are readily available and understood by all personnel.
Neutralization of Hydrochloric Acid
Neutralizing HCl spills or accidental exposures involves reducing its acidity by reacting it with a base. The most common base used for neutralization is sodium bicarbonate (baking soda).
Neutralization with Sodium Bicarbonate:
Sodium bicarbonate reacts with hydrochloric acid to produce sodium chloride (table salt), water, and carbon dioxide gas:
HCl (aq) + NaHCO3 (s) → NaCl (aq) + H2O (l) + CO2 (g)
The reaction is exothermic, meaning it releases heat. Therefore, it is important to add the sodium bicarbonate slowly to the acid to avoid excessive heat generation.
Other Neutralization Agents:
While sodium bicarbonate is the most common, other bases can also neutralize hydrochloric acid. These include:
- Sodium hydroxide (NaOH): A strong base that reacts quickly and efficiently with HCl. However, it generates significant heat, requiring careful handling.
- Calcium hydroxide (Ca(OH)2): A less corrosive base commonly used for neutralizing spills in industrial settings.
- Magnesium hydroxide (Mg(OH)2): A relatively safe and effective neutralizing agent found in milk of magnesia.
Spill Response Protocol
Prompt and efficient response is critical in the event of an HCl spill.
Steps to Take:
- Evacuate: Immediately evacuate the area and ensure all personnel are at a safe distance.
- Isolate: Isolate the spill area to prevent further contamination.
- Contain: If possible, contain the spill using absorbent materials like sand, vermiculite, or diatomaceous earth.
- Neutralize: Use an appropriate neutralizing agent like sodium bicarbonate, applying it slowly and carefully to the spill.
- Clean Up: Once neutralized, clean up the spill thoroughly using a mop or absorbent materials.
- Ventilation: Ensure adequate ventilation during and after cleanup to remove any remaining acid fumes.
- Disposal: Dispose of the neutralized spill and contaminated materials according to local regulations.
Important Considerations:
- Safety Gear: Always wear appropriate PPE when handling HCl or responding to spills.
- Ventilation: Ensure adequate ventilation to prevent exposure to acid fumes.
- Eye Wash and Shower: Have readily accessible eye wash stations and emergency showers in case of contact with HCl.
- Training: Provide regular training to personnel on safe handling procedures and spill response protocols.
FAQs
1. What happens if hydrochloric acid comes into contact with skin?
Contact with HCl can cause severe burns. Immediately flush the affected area with copious amounts of water for at least 15 minutes. Seek immediate medical attention.
2. What happens if hydrochloric acid is inhaled?
Inhaling HCl fumes can irritate the respiratory system, leading to coughing, wheezing, and difficulty breathing. Move to fresh air immediately. If symptoms persist, seek medical attention.
3. What are the signs of HCl poisoning?
Symptoms of HCl poisoning include burns in the mouth, throat, and stomach, vomiting, diarrhea, and abdominal pain. Seek immediate medical attention.
4. How do I dispose of hydrochloric acid safely?
Dispose of HCl according to local regulations. It is generally recommended to neutralize the acid before disposal.
5. Can I use vinegar to neutralize hydrochloric acid?
While vinegar (acetic acid) is acidic, it is not a suitable neutralizer for HCl. The reaction would generate heat and potentially harmful fumes.
Tips for Safe Handling of Hydrochloric Acid:
- Always read and understand the Material Safety Data Sheet (MSDS) for HCl before handling it.
- Store HCl in a secure, well-ventilated area, away from incompatible substances.
- Use appropriate PPE when handling HCl.
- Never mix HCl with other chemicals unless instructed by a qualified professional.
- Always work in a well-ventilated area.
- Ensure that emergency procedures for handling HCl spills are readily available and understood by all personnel.
Conclusion
Hydrochloric acid is a powerful and versatile chemical, but it requires careful handling and management. By adhering to safety precautions, understanding neutralization techniques, and implementing effective spill response protocols, individuals can minimize risks associated with HCl and ensure a safe working environment. Regular training, clear communication, and a proactive approach to safety are essential for mitigating the hazards of this important industrial chemical.








Closure
Thus, we hope this article has provided valuable insights into Managing Hydrochloric Acid: Safety Measures, Neutralization, and Spill Response. We appreciate your attention to our article. See you in our next article!