The Alkaline Universe of Your Home: Uncovering the Bases in Everyday Items
Related Articles: The Alkaline Universe of Your Home: Uncovering the Bases in Everyday Items
Introduction
With great pleasure, we will explore the intriguing topic related to The Alkaline Universe of Your Home: Uncovering the Bases in Everyday Items. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Alkaline Universe of Your Home: Uncovering the Bases in Everyday Items
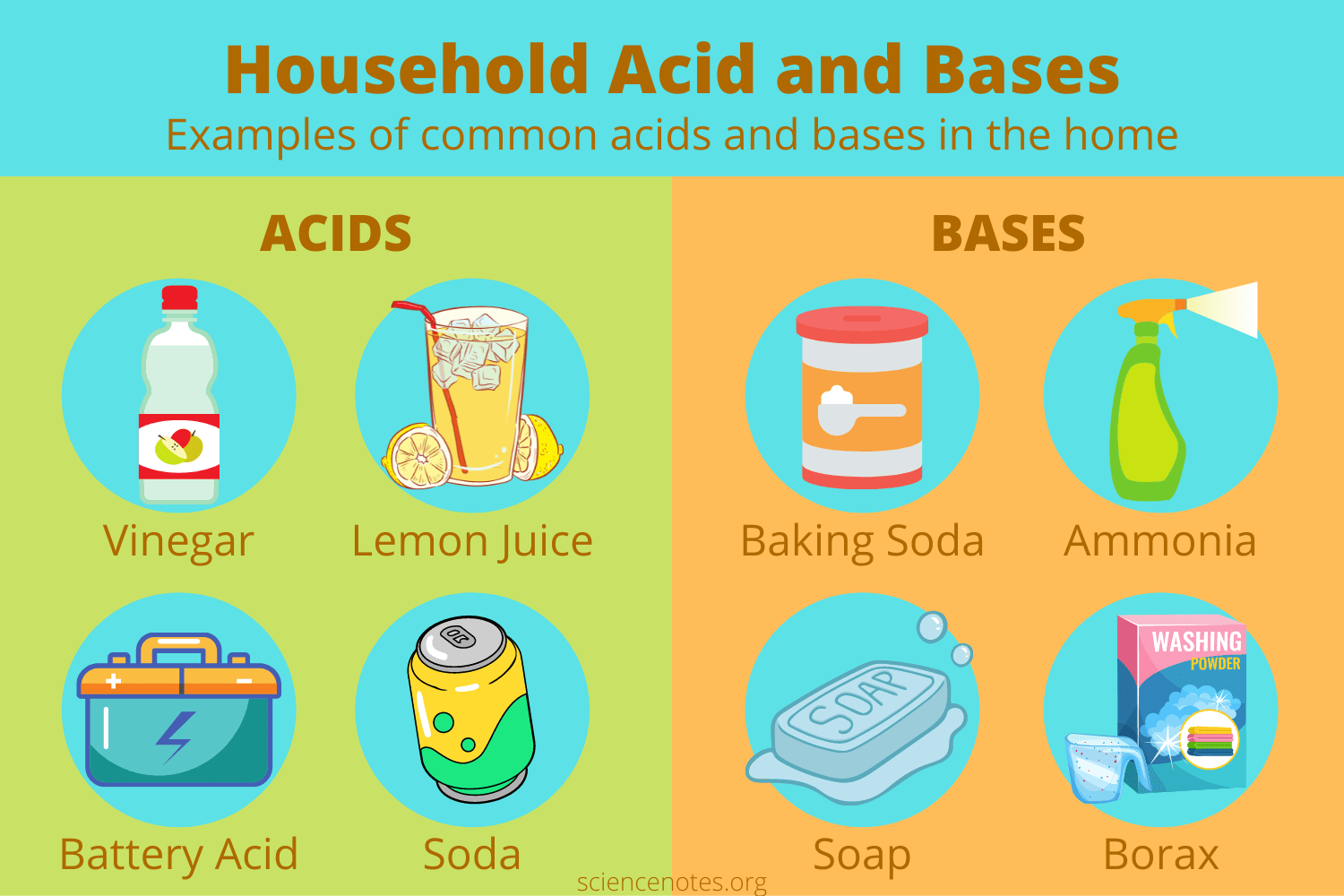
The world of chemistry often seems distant from the familiar comforts of our homes. However, a closer look reveals that the very items we use daily are teeming with chemical compounds, some of which exhibit basic properties. Bases, in the context of chemistry, are substances that release hydroxide ions (OH-) when dissolved in water. This release of hydroxide ions is what gives bases their characteristic properties, such as a slippery feel and the ability to neutralize acids. While most household items are acidic or neutral, a surprising number exhibit basic characteristics, playing a vital role in our daily lives.
Common Household Bases: Unveiling the Alkaline Side
1. Baking Soda (Sodium Bicarbonate):
Baking soda is a ubiquitous household item, primarily known for its leavening properties in baking. However, it is also a weak base, making it a versatile cleaning agent. The reaction of baking soda with acids, such as vinegar, produces carbon dioxide gas, creating a fizzing effect often used for cleaning and deodorizing. Baking soda’s mild alkalinity allows it to neutralize acidic spills, clean surfaces, and deodorize refrigerators.
2. Ammonia (NH3):
Ammonia is a colorless gas with a pungent odor, commonly used in household cleaners. It is a strong base, dissolving in water to form ammonium hydroxide, a highly alkaline solution. This strong alkalinity makes ammonia effective in dissolving grease and grime, making it a popular ingredient in window cleaners, floor cleaners, and all-purpose cleaners.
3. Washing Soda (Sodium Carbonate):
Washing soda, also known as soda ash, is a white powder commonly used as a laundry booster. It is a strong base, capable of softening hard water by removing calcium and magnesium ions. This softening action enhances the effectiveness of detergents, making it a valuable addition to laundry routines. Washing soda also finds applications in cleaning, particularly for removing grease and grime from surfaces.
4. Borax (Sodium Borate):
Borax is a naturally occurring mineral that is often used in laundry detergents and cleaning products. It is a weak base, known for its ability to soften water, remove stains, and disinfect surfaces. Borax’s mild alkalinity makes it a suitable ingredient for cleaning delicate fabrics and surfaces without causing damage.
5. Drain Cleaners:
Many drain cleaners contain strong bases like sodium hydroxide (lye) or potassium hydroxide. These highly alkaline substances effectively dissolve organic materials that clog drains, including hair, grease, and soap. However, it’s crucial to use drain cleaners with caution due to their corrosive nature.
6. Antacids:
Antacids are medications designed to neutralize excess stomach acid, providing relief from heartburn and indigestion. Common antacids like calcium carbonate and magnesium hydroxide are bases that react with stomach acid, reducing its acidity and relieving symptoms.
7. Soaps:
Soaps are salts of fatty acids, formed by reacting fats or oils with strong bases like sodium hydroxide or potassium hydroxide. The resulting soap molecules have a polar head (hydrophilic) and a nonpolar tail (hydrophobic), allowing them to emulsify grease and dirt, making them effective cleaning agents.
Benefits of Household Bases:
1. Cleaning and Deodorizing:
The alkalinity of bases allows them to effectively break down grease, grime, and organic matter, making them essential for cleaning and deodorizing surfaces.
2. Water Softening:
Bases like washing soda can soften hard water by removing calcium and magnesium ions, improving the effectiveness of detergents and preventing mineral buildup in pipes.
3. Neutralizing Acids:
Bases react with acids to neutralize them, making them useful for treating acidic spills, neutralizing stomach acid, and controlling pH levels.
4. Disinfecting:
Some bases, such as borax and ammonia, possess antimicrobial properties, making them effective disinfectants for surfaces and materials.
5. Industrial Applications:
Bases find numerous applications in various industries, including manufacturing, construction, and agriculture.
FAQs about Household Bases:
Q: Are all household bases safe to use?
A: No, not all household bases are safe to use. Strong bases like lye and potassium hydroxide can be corrosive and dangerous if mishandled. Always read product labels carefully and follow safety instructions.
Q: What are the risks associated with using household bases?
A: Bases can cause skin irritation, eye damage, and respiratory problems if mishandled. Always wear gloves and eye protection when using bases, and ensure adequate ventilation.
Q: How do I dispose of household bases safely?
A: Never pour bases down the drain, as they can damage pipes. Check with your local waste management facility for proper disposal guidelines.
Q: Can I mix different household bases together?
A: Mixing different bases can create dangerous reactions. Avoid mixing bases unless specifically instructed by a product label.
Q: What happens when a base reacts with an acid?
A: When a base reacts with an acid, they neutralize each other, forming salt and water. This reaction releases heat, which can be significant in some cases.
Tips for Using Household Bases Safely:
- Always read product labels carefully and follow instructions.
- Wear gloves and eye protection when handling bases.
- Ensure adequate ventilation when using bases.
- Keep bases away from children and pets.
- Store bases in their original containers and label them clearly.
- Never mix bases with acids unless specifically instructed by a product label.
- Dispose of bases properly according to local waste management guidelines.
Conclusion:
Household bases are an integral part of our daily lives, playing a crucial role in cleaning, deodorizing, and maintaining our homes. While they offer numerous benefits, it’s essential to use them safely and responsibly. By understanding the properties of bases and following safety precautions, we can harness their power to enhance our lives while minimizing potential risks.







Closure
Thus, we hope this article has provided valuable insights into The Alkaline Universe of Your Home: Uncovering the Bases in Everyday Items. We appreciate your attention to our article. See you in our next article!
