The Spectrum of Light: Unveiling the Sources of Illumination
Related Articles: The Spectrum of Light: Unveiling the Sources of Illumination
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to The Spectrum of Light: Unveiling the Sources of Illumination. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Spectrum of Light: Unveiling the Sources of Illumination
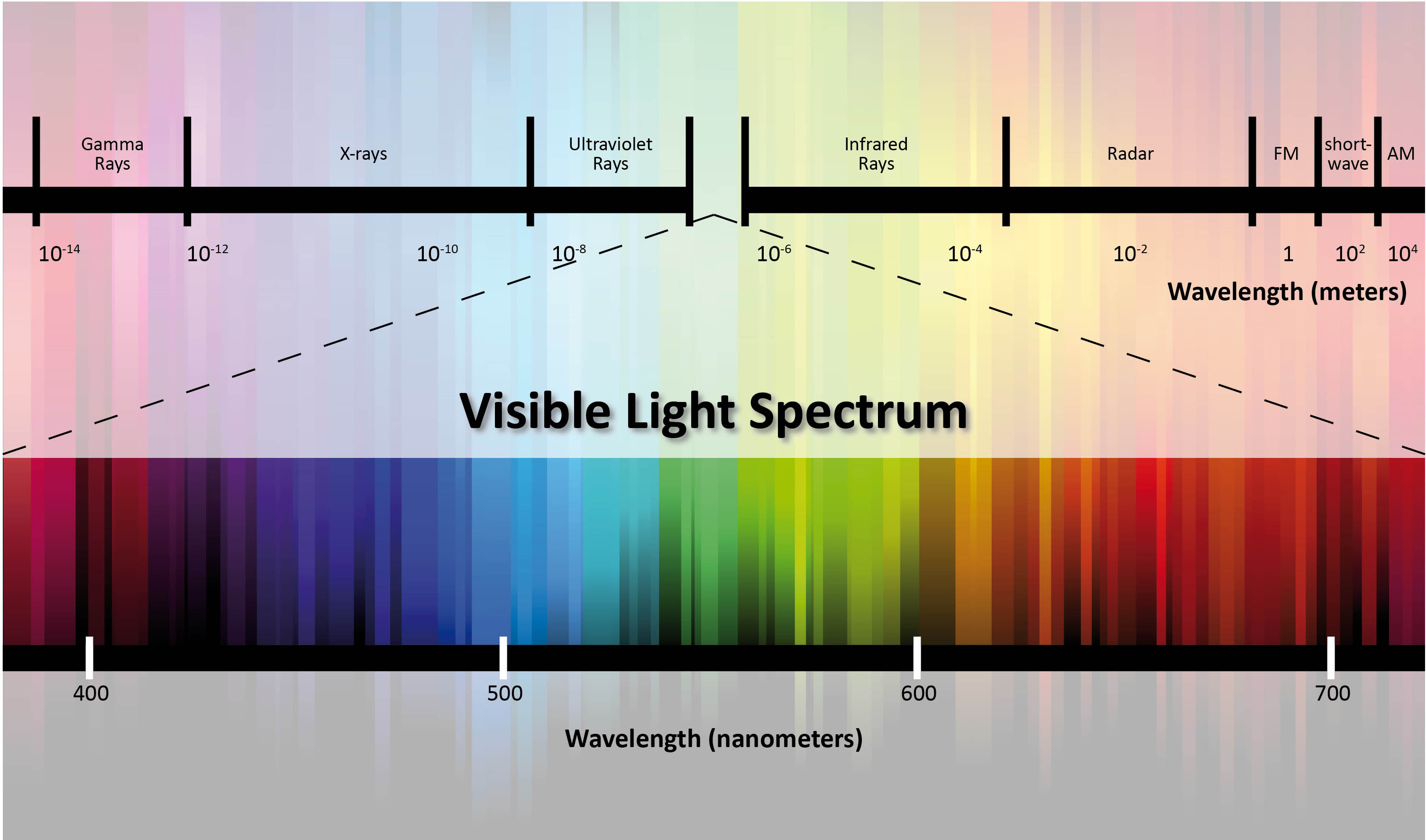
/the-visible-light-spectrum-2699036_FINAL2-c0b0ee6f82764efdb62a1af9b9525050.png)
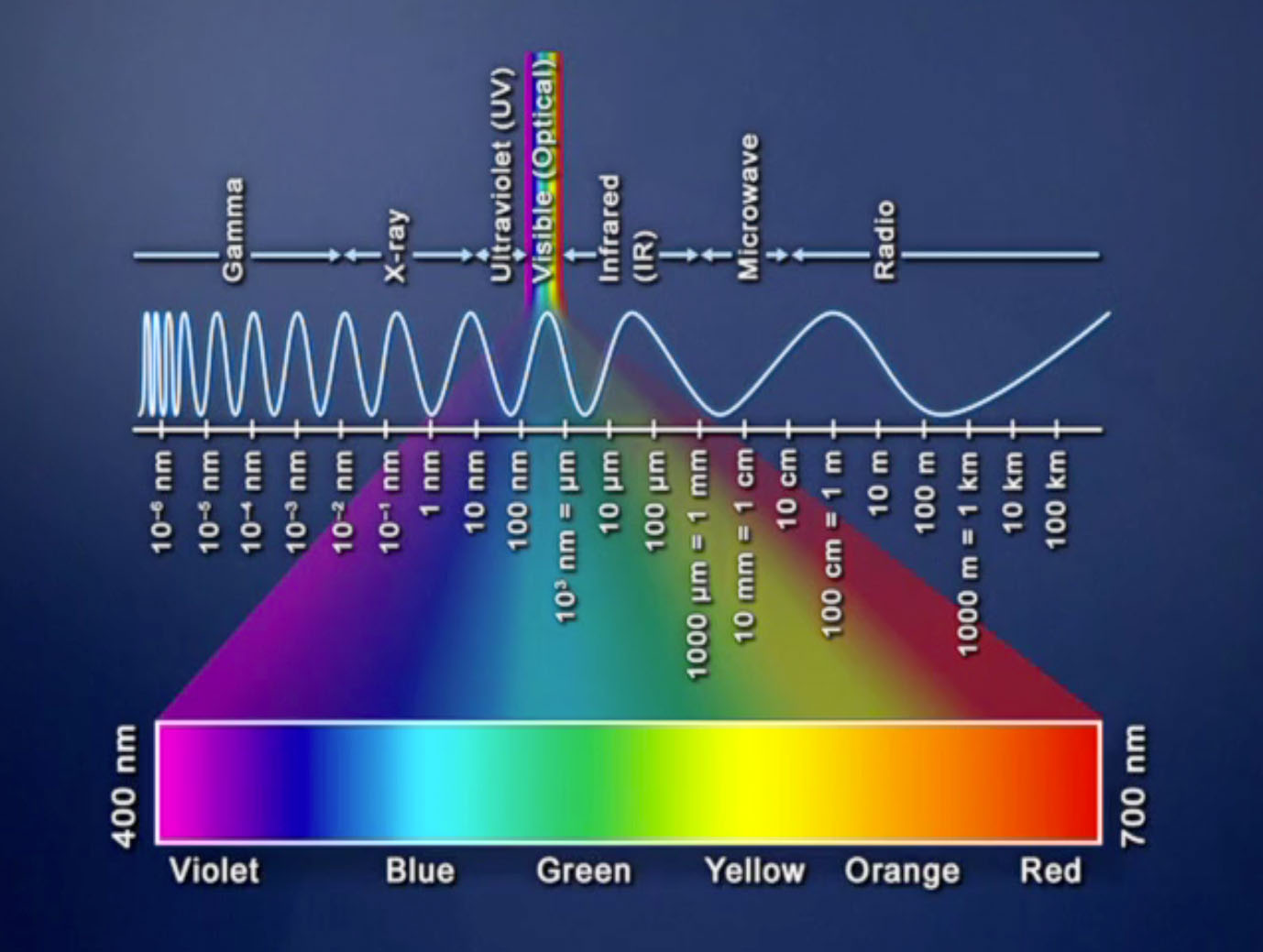
The world around us is bathed in a sea of electromagnetic radiation, a spectrum of energy waves that range from the invisible to the visible. Among these waves, light, with its familiar wavelengths, plays a pivotal role in our perception of the world and in countless technological applications. This article delves into the fascinating realm of light emission, exploring the diverse mechanisms by which objects produce these illuminating waves.
The Fundamental Physics of Light Emission
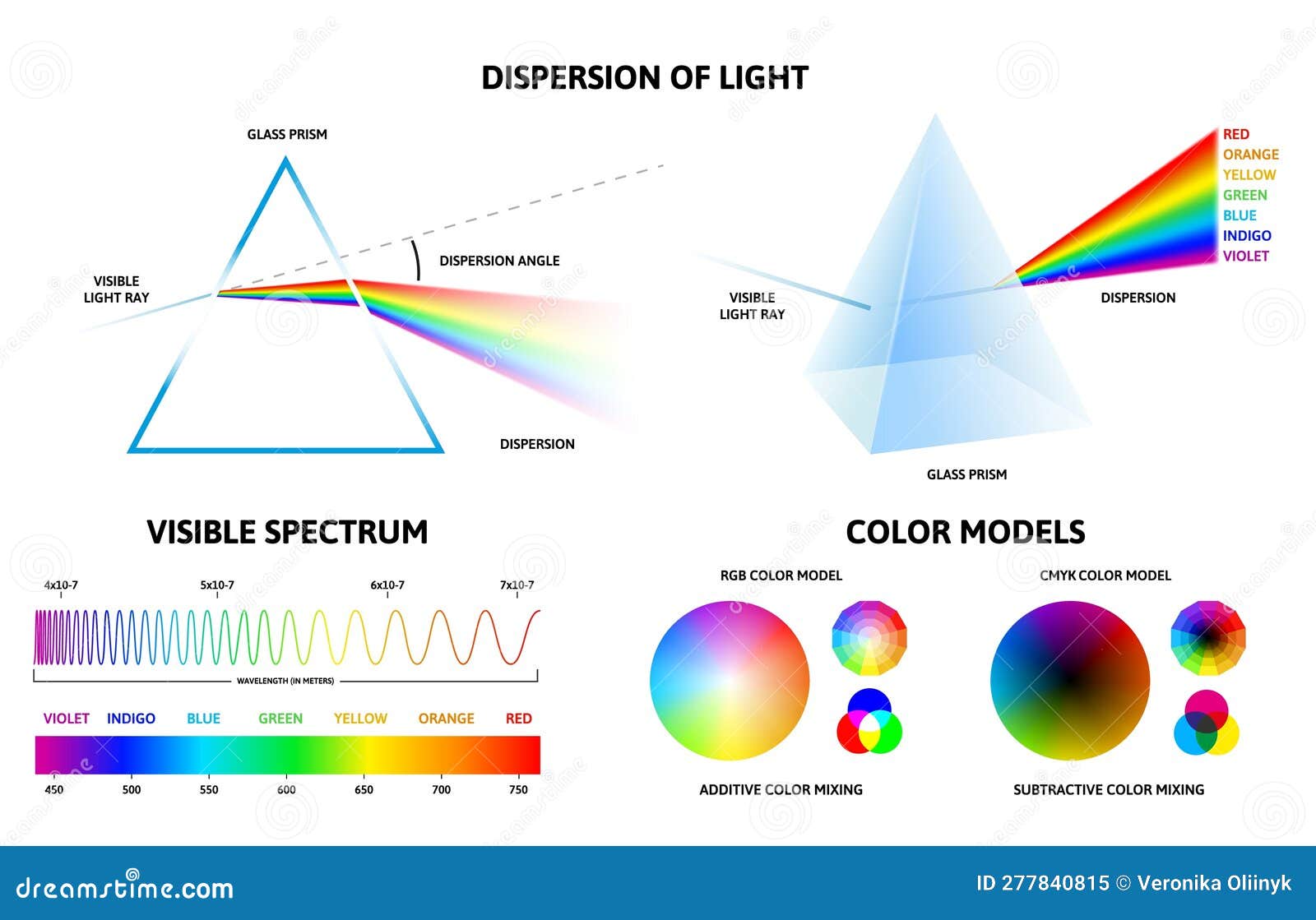
At the heart of light emission lies the fundamental concept of excited atoms. When an atom absorbs energy, its electrons transition to higher energy levels, a state known as excitation. This excited state is unstable, and the atom seeks to return to its ground state, the lowest energy level. This transition is accompanied by the release of energy in the form of a photon, a particle of light. The energy of the emitted photon directly corresponds to the difference in energy between the excited and ground states, determining the wavelength and color of the light.
Natural Light Sources: The Sun and Stars
The most prominent natural source of light is the Sun, a massive star fueled by nuclear fusion. In the Sun’s core, hydrogen atoms fuse to form helium, releasing an immense amount of energy in the form of light and heat. This radiant energy travels through space and reaches Earth, providing the energy necessary for life and driving countless natural processes.
Stars, like the Sun, also emit light through nuclear fusion, albeit at varying intensities and temperatures. The color of a star is directly related to its temperature: hotter stars emit bluer light, while cooler stars emit redder light. This stellar light, traveling across vast cosmic distances, allows us to study the composition and evolution of the universe.
Incandescent Light: Heating Up to Glow
Incandescent light bulbs, a familiar fixture in homes for over a century, operate on the principle of thermal radiation. When an electric current flows through a filament, usually made of tungsten, it heats up to extremely high temperatures. This heat excites the atoms within the filament, causing them to emit light across a broad spectrum, including visible light and infrared radiation.
Despite their familiarity, incandescent bulbs are relatively inefficient, converting only a small fraction of electrical energy into visible light. The majority of the energy is dissipated as heat, making them energy-intensive and contributing to environmental concerns.
Fluorescent Light: Harnessing Electron Excitation
Fluorescent lighting, a more efficient alternative to incandescent bulbs, relies on the principle of fluorescence. A gas, typically mercury vapor, is excited by an electric current, causing its atoms to emit ultraviolet (UV) radiation. This UV light then strikes a phosphor coating on the inside of the bulb, causing it to emit visible light.
Fluorescent bulbs offer improved energy efficiency compared to incandescent bulbs, but they contain mercury, a toxic substance requiring careful disposal.
LED Lighting: The Future of Illumination
Light-emitting diodes (LEDs) have revolutionized lighting technology, offering exceptional energy efficiency, long lifespan, and versatility. LEDs operate by passing an electric current through a semiconductor material, typically gallium nitride or gallium arsenide. This current causes electrons to transition to higher energy levels, and when they return to their ground state, they emit photons of light.
LEDs exhibit several advantages over traditional lighting technologies. They are highly energy efficient, converting a significant portion of electrical energy into visible light. They also have a much longer lifespan than incandescent and fluorescent bulbs, reducing the need for frequent replacements. Furthermore, LEDs are available in a wide range of colors and can be easily dimmed, offering flexibility for various applications.
Other Light-Emitting Phenomena
Beyond these common sources, various other phenomena contribute to light emission:
- Chemiluminescence: Chemical reactions can produce light, as seen in glow sticks, where a chemical reaction releases energy that excites molecules, causing them to emit light.
- Bioluminescence: Some living organisms, such as fireflies and jellyfish, produce light through chemical reactions within their bodies. This phenomenon serves various purposes, including attracting mates, deterring predators, or illuminating the deep sea.
- Phosphorescence: Certain materials, after absorbing light energy, continue to emit light for a period of time even after the light source is removed. This phenomenon is used in glow-in-the-dark toys and emergency exit signs.
- Lasers: Lasers, an acronym for "Light Amplification by Stimulated Emission of Radiation," generate highly focused beams of monochromatic light. They utilize stimulated emission, a process where excited atoms are stimulated to emit photons in phase, resulting in a coherent and powerful beam of light. Lasers find applications in diverse fields, including telecommunications, medicine, and manufacturing.
The Importance of Light Emission
Light emission plays a crucial role in our lives and in countless technological applications:
- Vision: Light is the foundation of our ability to see. Light waves enter our eyes, stimulating photoreceptor cells in the retina, which transmit signals to the brain, allowing us to perceive the world around us.
- Communication: Light is used in fiber optic cables for high-speed data transmission, enabling internet access and communication networks.
- Medical Applications: Light is used in medical imaging techniques like X-rays and MRI scans, allowing doctors to diagnose and treat diseases. Lasers are also employed in various surgical procedures and for treating skin conditions.
- Agriculture: Artificial lighting can be used to extend the growing season for crops, increasing food production and promoting year-round harvests.
- Entertainment: Light is essential for theatrical performances, concerts, and other forms of entertainment, creating immersive experiences and enhancing visual effects.
FAQs about Light Emission
Q: What is the difference between light and heat?
A: Light and heat are both forms of electromagnetic radiation, but they differ in their wavelengths. Light refers to the visible portion of the electromagnetic spectrum, while heat encompasses a broader range of wavelengths, including infrared radiation.
Q: Can all objects emit light?
A: While all objects emit some form of electromagnetic radiation, not all objects emit visible light. The temperature of an object determines the wavelengths of radiation it emits. Objects at room temperature primarily emit infrared radiation, which is invisible to the human eye. Only objects heated to high temperatures emit visible light.
Q: How does a laser differ from a regular light source?
A: Lasers emit a highly focused beam of monochromatic light, meaning all the photons have the same wavelength and travel in the same direction. Regular light sources emit light across a broad spectrum of wavelengths and in random directions.
Q: What are the environmental impacts of different lighting technologies?
A: Incandescent bulbs are energy-intensive and produce significant heat, contributing to greenhouse gas emissions. Fluorescent bulbs contain mercury, a toxic substance requiring careful disposal. LEDs are highly energy efficient and have a long lifespan, reducing energy consumption and waste.
Tips for Using Light Efficiently
- Choose energy-efficient lighting: Opt for LED bulbs, which offer significant energy savings compared to traditional incandescent and fluorescent bulbs.
- Use natural light: Maximize the use of natural light during the day by positioning furniture and workspaces near windows.
- Dim the lights: Dim the lights when not needed to reduce energy consumption and create a more relaxing atmosphere.
- Turn off lights when leaving a room: This simple habit can significantly reduce energy waste.
- Use timers and motion sensors: Install timers for outdoor lighting and motion sensors for indoor lighting to automatically turn lights on and off when needed.
Conclusion
The world of light emission is a fascinating realm of physics and technology. From the Sun’s radiant energy to the intricate workings of LEDs, the ability to produce light has shaped our understanding of the universe and our daily lives. As technology advances, we can expect even more innovative and efficient light sources to emerge, illuminating our future with brighter possibilities.





Closure
Thus, we hope this article has provided valuable insights into The Spectrum of Light: Unveiling the Sources of Illumination. We appreciate your attention to our article. See you in our next article!