Understanding Borax: A Comprehensive Look At Its Composition And Applications
Understanding Borax: A Comprehensive Look at its Composition and Applications
Related Articles: Understanding Borax: A Comprehensive Look at its Composition and Applications
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Understanding Borax: A Comprehensive Look at its Composition and Applications. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Understanding Borax: A Comprehensive Look at its Composition and Applications
/what-is-borax-where-to-get-608509_FINAL-277034df3e8447dea95cf03285b01155.png)
Borax, also known as sodium borate, sodium tetraborate, or disodium tetraborate, is a naturally occurring mineral compound with a wide range of applications. It is a white, crystalline substance that is readily soluble in water, forming a mildly alkaline solution. While its chemical formula is Na2B4O7·10H2O, understanding its constituent parts and their properties is crucial to appreciating its diverse uses.
The Chemical Makeup of Borax:
Borax’s chemical composition reveals its key components:
- Sodium (Na): An alkali metal that readily forms ionic bonds. It contributes to borax’s solubility and its ability to interact with various substances.
- Boron (B): A metalloid element that forms the borate ion (BO33-). This ion is responsible for borax’s unique chemical properties, including its ability to act as a weak base and form complexes with other compounds.
- Oxygen (O): A highly electronegative element that forms the basis of the borate ion and contributes to the overall structure of the borax molecule.
- Water (H2O): Borax exists in a hydrated form, meaning it incorporates water molecules into its crystal structure. The number of water molecules can vary, with the decahydrate (Na2B4O7·10H2O) being the most common form. These water molecules contribute to borax’s solubility and its ability to release heat when dissolved.
Understanding the Borate Ion:
The borate ion (BO33-) is the key to borax’s unique properties. It is a triangular planar structure with boron at the center and oxygen atoms at the vertices. This structure allows the borate ion to form complex compounds with other molecules, including metals, sugars, and organic acids.
Borax’s Versatile Applications:
Borax’s diverse applications stem from its chemical properties:
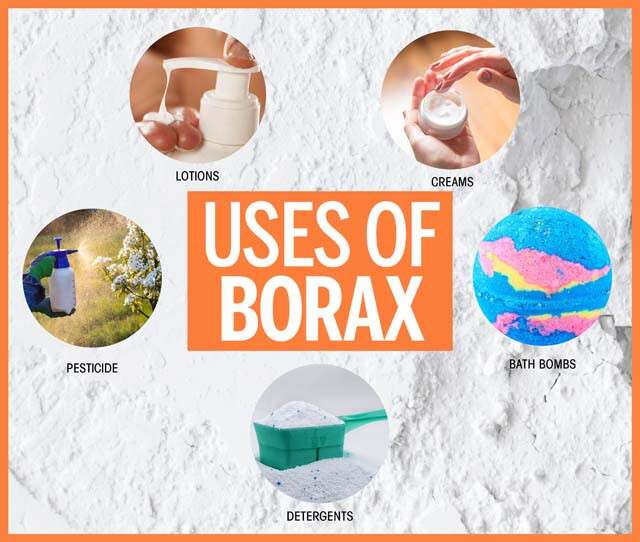
- Detergent and Cleaning Agent: Borax is a natural cleaning agent due to its mildly alkaline nature. It can be used to clean laundry, dishes, and surfaces, and it is also effective in removing stains. Borax’s ability to form complexes with metal ions helps it to soften water and improve the effectiveness of detergents.
- Pesticide and Insecticide: Borax’s toxicity to insects has led to its use as a natural insecticide, particularly for controlling cockroaches, ants, and other pests. It works by disrupting the insects’ digestive systems.
- Antiseptic and Disinfectant: Borax’s antimicrobial properties have been recognized for centuries. It can be used to disinfect wounds, surfaces, and water. Its effectiveness against bacteria and fungi makes it a valuable component in various hygiene products.
- Fire Retardant: Borax’s ability to absorb heat and release water vapor makes it effective as a fire retardant. It is often incorporated into fabrics, wood, and other materials to slow the spread of fire.
- Glass and Ceramics: Borax is a crucial ingredient in the manufacturing of glass and ceramics. It acts as a flux, lowering the melting point of the raw materials and allowing for smoother processing.
- Agriculture: Borax is used in agriculture as a micronutrient for plants. It is essential for the growth and development of certain crops, particularly those that require boron for optimal photosynthesis and cell wall formation.
- Industrial Uses: Borax is used in various industrial applications, including the manufacture of enamels, paints, and adhesives. Its ability to form complexes with metal ions makes it useful in metal processing and refining.
Safety Considerations:
While borax has numerous benefits, it is important to use it responsibly and with caution. It is considered mildly toxic when ingested in large quantities and can cause skin irritation or allergic reactions in some individuals. Always follow the manufacturer’s instructions and wear appropriate protective gear when handling borax.
FAQs:
Q: Is borax safe to use in cleaning products?
A: Borax is generally considered safe for cleaning purposes when used in recommended concentrations. However, it is important to avoid direct contact with eyes, skin, and mucous membranes.
Q: Is borax harmful to pets?
A: Borax can be toxic to pets, especially dogs and cats. If ingested, it can cause digestive upset, vomiting, diarrhea, and even death. Keep borax products out of reach of pets.
Q: Can borax be used to kill mold?
A: Borax can be effective in controlling mold growth, but it is not a primary treatment for mold infestations. It is best used in conjunction with other mold removal methods.
Q: Is borax a natural product?
A: Borax occurs naturally as a mineral, but it is also produced synthetically.
Tips for Using Borax:
- Always follow the manufacturer’s instructions for the specific product.
- Wear gloves and eye protection when handling borax.
- Keep borax products out of reach of children and pets.
- Do not mix borax with other cleaning products unless specifically instructed.
- Store borax in a cool, dry place.
Conclusion:
Borax, a naturally occurring mineral compound, holds a significant place in various industries and households due to its unique chemical properties. Its ability to act as a cleaning agent, insecticide, antiseptic, fire retardant, and flux makes it a versatile material with diverse applications. Understanding its composition, properties, and safety considerations is crucial for utilizing its benefits responsibly and effectively. While borax offers numerous advantages, it is important to use it with caution and follow recommended guidelines to ensure safety and minimize potential risks.





Closure
Thus, we hope this article has provided valuable insights into Understanding Borax: A Comprehensive Look at its Composition and Applications. We appreciate your attention to our article. See you in our next article!
